By: Ankita Kulkarni
May 11 2021
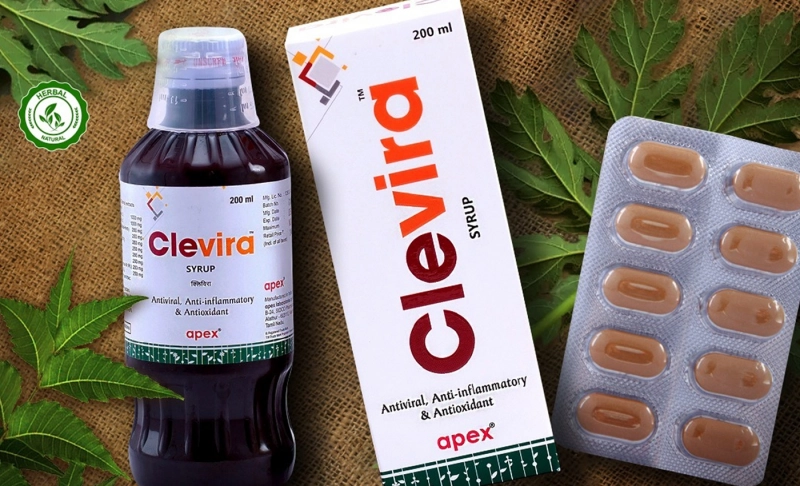
Partly True: An ayurvedic anti-viral drug named Clevira is used to treat mild and moderate cases of COVID-19.
The Verdict Partly_True
Clevira is approved by CCRAS and ITRC as a supporting drug to boost immunity and platelets count in treating mild and moderate COVID-19 cases.
Clevira is approved by CCRAS and ITRC as a supporting drug to boost immunity and platelets count in treating mild and moderate COVID-19 cases.Clevira is an antiviral ayurvedic drug that was previously used to treat dengue and is now approved as a supportive treatment for COVID-19 patients with mild and moderate symptoms. The antiviral drug is manufactured by Apex Laboratories Private Limited in Tamil Nadu. It proved effective when taken orally as a tablet dosage twice a day for 14 days after food. It is proven to be safe for the kidney and liver. The clinical trial included 100 randomized participants divided into two groups of 50 individuals each. A 30 day-trial conducted at Government Medical College Omandurar and Government Estate in Chennai revealed that test groups that received Clevira were able to recover from pyrexia clinically or body pain. It also normalized the respiratory rate and improved oxygen saturation level from day 5 to 15 of testing as per primary and secondary outcomes published in the Clinical Trials Registry of India. The results of the clinical trials were submitted to the Tamil Nadu government, ICMR (Indian Council of Medical Research), and the Ministry of AYUSH in 2020. The New Indian Express reported that the company noted that “after rigorous scrutiny and deliberations, the drug was approved as a supporting measure by the Central Council for Research in Ayurvedic Sciences (CCRAS) and the Interdisciplinary Technical Review Committee (ITRC) along with normal treatment for patients experiencing mild to moderate COVID-19 symptoms.” The report further quoted that C Arthur Paul, international business manager at Apex Laboratories. He said that the drug successfully improved the platelet counts and increased white blood cells, which boost the recovery time in COVID-19 patients. The antiviral drug is only approved as a supportive treatment in hospitals but not as an exclusive medicine against the coronavirus. The COVID-19 pandemic has given rise to a lot of potentially dangerous misinformation. For reliable advice on COVID-19 including symptoms, prevention and available treatment, please refer to the World Health Organisation or your national healthcare authority.


